
Real-world evidence, or RWE, is fundamentally changing the future of healthcare. Here’s how it happens:
- Real-world data (RWD) is aggregated and transformed into RWE through robust analytics.
- Real-world evidence (RWE) provides clinically-rich insights into what actually happens in everyday practice and why.
- Stakeholders across the healthcare ecosystem use this new knowledge to support decision-making and improve safety and effectiveness, and ultimately, patient outcomes.
Let’s take a closer look at RWD, how it becomes RWE, and the value of RWE across the healthcare ecosystem delivered through nine use cases.
In this post we will cover:
- What Is Real-World Data (RWD)?
- What Is Real-World Evidence (RWE)?
- Why Do We Need Real-World Evidence?
- Use of Real-World Evidence in Pharmaceutical and Device Companies
- Use of Real-World Evidence Among Regulators
- Use of Real-World Evidence Among Clinicians and Academic Researchers
- Use of Real-World Evidence in Healthcare Systems
- The Role of Registries in Generating Real-World Evidence
What Is Real-World Data (RWD)?
In order to understand real-world evidence and its role in healthcare, we must first define real-world data, or RWD.
Real-world data is any data that is collected in the context of the routine delivery of care, as opposed to data collected within a clinical trial where study design controls variability in ways that are not representative of real-world care and outcomes.
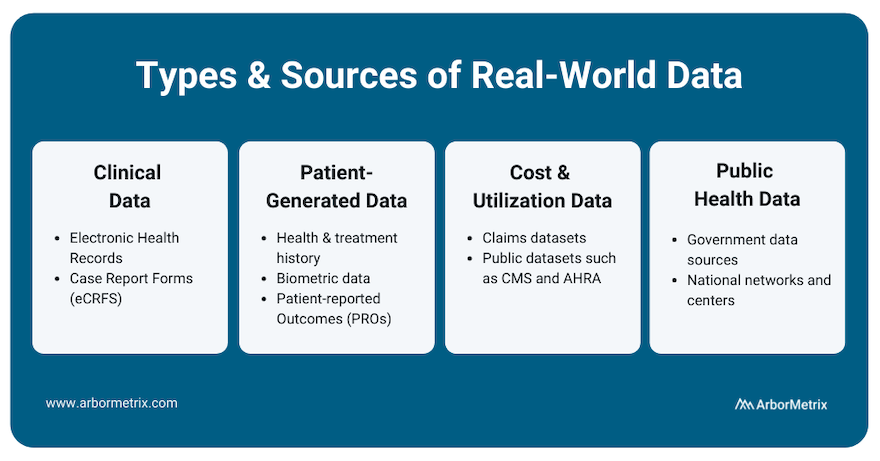
There are many different types and sources of RWD. Let’s take a closer look at each type, its source, and its uses.
- Clinical data from electronic health records (EHRs) and case report forms (eCRFs). This data provide patient demographics, family history, comorbidities, procedure and treatment history, and outcomes.
- Patient-generated data from patient-reported outcome (PRO) surveys. These data provide insights directly from the patient, and they help researchers understand what happens outside of clinic visits, procedures, and hospital stays.
- Cost and utilization data from claims and public datasets. They provide information regarding healthcare services utilization, population coverage, and prescribing patterns.
- Public health data from various government data sources. These add critical information to enable stakeholders to best serve the needs of the populations they serve.
What Is Real-World Evidence (RWE)?
The U.S. Food and Drug Administration (FDA) defines RWE as “the clinical evidence regarding the usage and potential benefits or risks of a medical product derived from analysis of real-world data.” [1]
Unlike traditional clinical trials, where necessary data elements can be curated and collection mandated, the creation of RWE requires assessing, validating and aggregating various, often disparate, sources of data available through routine clinical practice.
The creation of RWE requires a combination of high-powered analytics, a validated approach and a robust knowledge of available RWD sources (e.g. what data is captured within existing quality registries, what data can be captured through an EHR or claims, which patient organizations capture data on relevant patient cohorts).
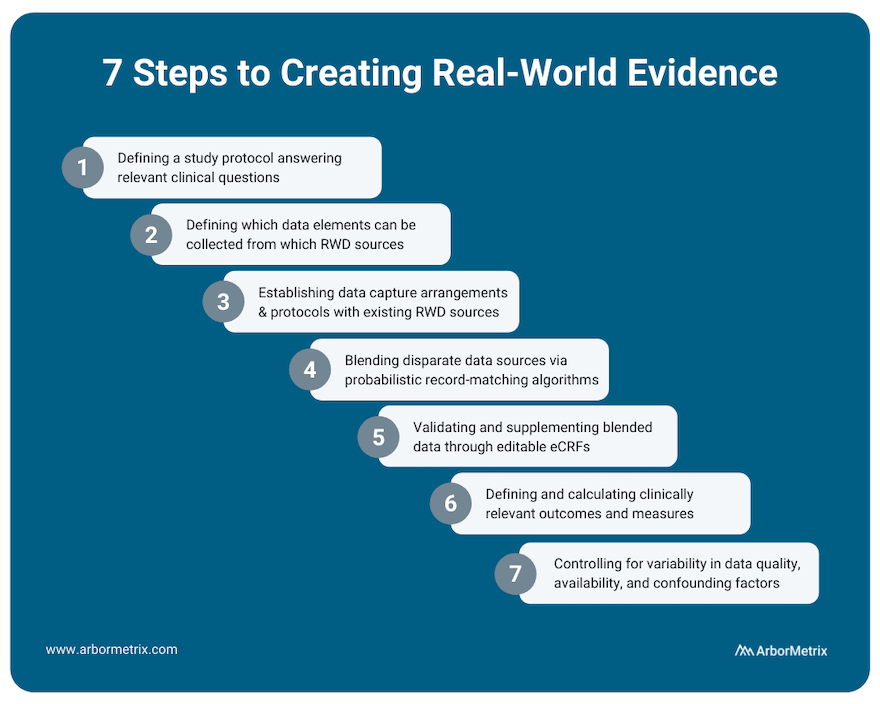
This process includes several steps:
- Defining a study protocol answering relevant clinical questions.
- Defining which data elements can be collected from which RWD sources.
- Establishing data capture arrangements and protocols with existing RWD sources.
- Blending disparate data sources through probabilistic record matching algorithms.
- Validating and supplementing blended data through editable eCRFs.
- Defining and calculating clinically relevant outcomes and measures.
- Appropriately assessing and controlling for variability in data quality, availability and confounding patient factors affecting measured outcomes.
RWE can provide a holistic view of patients that in many cases cannot be studied through traditional clinical trials.
Why Do We Need Real-World Evidence?
There’s a gap between research (what we learn) and everyday practice (what we do) in healthcare, and it creates a difference between what is expected to happen and what really happens.
But it’s what really happens that matters. Driving measurable improvements in healthcare requires us all to be rooted in the reality of what actually happens before, during, and after clinical procedures, interventions, and office visits.
RWE is here to fill those gaps and root us in truth. It tells us what really happens when doctors treat a wide range of patients that don’t look like the homogeneous patient groups in a clinical trial.
Because of this, RWE serves many uses and provides many benefits across the healthcare ecosystem.
Uses of Real-World Evidence in Pharmaceutical and Device Companies
Pharmaceutical and medical device companies are major consumers of RWE, as it can provide value across the entire product lifecycle.
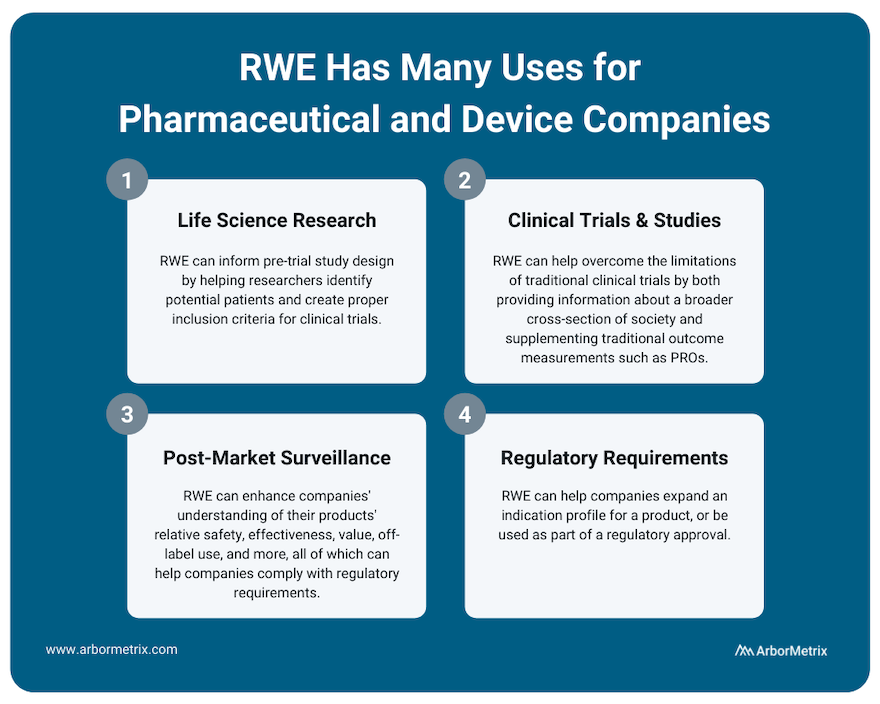
Use Case #1: RWE and Life Science Research (Pre-Trial Design)
RWE plays an important role for research across the product lifecycle for both pharmaceutical and device companies. It can inform pre-trial study design by helping researchers identify potential patients and create proper inclusion criteria for clinical trials.
Use Case #2: RWE and Clinical Studies and Trials
Much of medical innovation is driven by traditional clinical trials, where new pharmaceuticals and devices are rigorously studied and tracked before they can be sold and widely distributed. Although clinical trials are incredibly important to determine the safety and efficacy of new technologies, when compared to real-world evidence they do have some limitations.
For example:
- Traditional clinical trials can have strict inclusion criteria that makes it challenging for providers to accurately extrapolate the results of a clinical trial to a broader population.
- Clinical trial participation is often limited by who the study administrators are able to recruit, and various demographics are often not able to participate. This again challenges the generalizability of clinical trial results across patient populations.
Real-world evidence can help overcome the limitations of clinical trials by providing information about a broader cross-section of society. This can help clinicians, researchers, and industry partners better understand their products and how they work.
Also, during clinical trials, RWE can supplement traditional outcome measurements with patient-generated data such as patient-reported outcomes (PROs) or biometric data that is easier to collect between doctor appointments.
Use Case #3: RWE and Post-Market Surveillance
Once a product is approved and marketed, RWE can help a pharmaceutical or medical device company understand their products’ relative safety, effectiveness, value, off-label use and more. This post-market surveillance, or post-marketing surveillance, is valuable to stakeholders across healthcare. For example, we support several clinical data registries that provide real-time RWE that device manufacturers use to evaluate their products.
Purposes of this post-market surveillance may include:
- Detecting adverse events or risks as they arise during real-world usage of a device or drug.
- Comparing new products or treatments with existing options and the standard of care.
- Updating clinical guidelines as certain populations or groups find more benefit than others.
- Complying with regulatory requirements.
Use Case #4: RWE and Regulatory Requirements
RWE can help companies expand an indication profile for a product. For example, while it may not be feasible to perform a full clinical trial for a product that is commonly used for off-label indications, by collecting RWD from disease or product registries, companies may be able to study safety and outcomes data for their product or device, which can then be used to supplement a submission to the U.S. FDA or European Medicines Agency (EMA).
In addition, the U.S. FDA has indicated that for certain products, a promise of RWE analysis in the post-market period can be used as part of a regulatory approval. For example, it might not be feasible to run a clinical trial on a product that is to be implanted for a long period of time. In a case like this, post-market RWE can help provide that missing long-term data.
Uses of Real-World Evidence Among Regulators
Regulatory bodies like the U.S. FDA and the EMA are increasingly using real-world evidence, and in some cases requiring it, to satisfy post-market approvals.
Use Case #5: RWE and Post-Market Approvals
The U.S. FDA has provided a framework that provides insight and instruction on how to utilize RWE to support a new drug or device regulatory filing. [2]
They specifically note a few use cases where RWD and RWE can be utilized:
- Expanded label indications for a product or device.
- Post-market surveillance studies.
- Post-approval device surveillance as a condition of approval.
- Control groups for certain clinical studies.
- Supplementary data to help understand clinical trial data.
- Objective performance criteria and performance goals.
Uses of Real-World Evidence Among Clinicians and Academic Researchers
Use Case #6: RWE and Decision Support Tools
Through advanced analytics, RWE can provide individualized decision support tools that can facilitate shared decision-making between a patient and physician. For example, the Michigan Bariatric Surgery Collaborative uses a tool that predicts, based on individual characteristics, how a patient might respond to different types of bariatric surgery.
Use Case #7: RWE and Guideline Development
Through comparing outcomes for different treatment options, it might become evident that particular drugs or dosages are better optimized for different individuals, and treatment guidelines can be refined and optimized accordingly.
Use Case #8: RWE and Clinical Research
With large real-world datasets, researchers can perform observational, retrospective, and prospective studies that help academia advance its understanding of current therapies and technologies. Check out this list of research publications that use RWE from clinical registries to advance knowledge.
Uses of Real-World Evidence in Healthcare Systems
Use Case #9: Quality Measurement, Benchmarking, and Improvement
RWE can track outcome and quality measures across organizations. For administrators, RWE can provide information about how their entire health system is performing relative to others, and how their individual physicians are benchmarking relative to peers. This information can help inform coverage discussions with payers, and refine quality-based reimbursement strategies that reward solid outcomes.
The Role of Registries in Generating Real-World Evidence
Clinical registries, including patient, product and claims registries, not only provide a rich source of RWD. They are also critical tools for generating RWE itself and using RWE to support post-market surveillance and research.
